Product Usage: This product is designated as a "Novelty Peptide" and is intended solely for specialized applications in controlled settings. It is not intended for any specific biological, therapeutic, or diagnostic use. All information provided on this website is for informational and educational purposes only and should not be construed as guidance for any particular application or usage.
What Is ARA-290 16MG?
ARA-290 is a synthetic peptide derived from the tertiary structure of erythropoietin (EPO). It is specifically designed to activate the innate repair receptor (IRR), which mediates tissue protection and repair without the erythropoietic effects of EPO. ARA-290 is a small, 11-amino acid peptide with a molecular weight of 1257 daltons that mimics the spatial configuration of the B-helix of the EPO molecule. This peptide has shown promising results in reducing neuropathic pain, promoting tissue repair, and improving metabolic control in various preclinical and clinical studies.
Structure
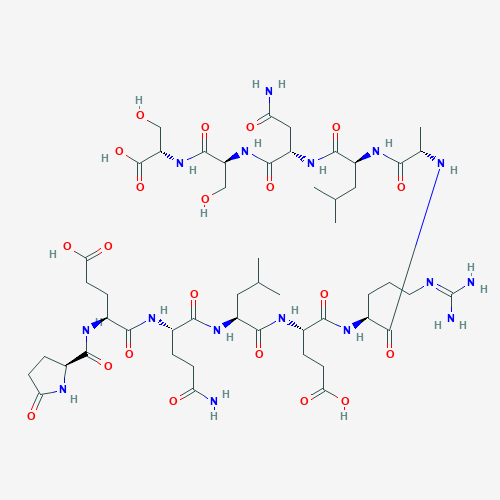
Sequence:XEQLERALNSS
Molecular Formula:C51H84N16O21
Molecular Weight:1257 Daltons
CAS Number:1208243-50-8
Synonyms: Cibinetide, PHBSP
Research Findings
- Neuropathic Pain Relief: ARA-290 has demonstrated significant efficacy in reducing mechanical and cold allodynia in animal models of neuropathic pain. In a study using the spared nerve injury model, ARA-290 produced long-lasting relief of tactile allodynia in a dose-dependent manner.
- Small Fiber Neuropathy (SFN) Symptom Improvement: Clinical trials in patients with sarcoidosis-associated SFN showed that ARA-290 significantly improved symptoms as measured by the Small Fiber Neuropathy Screening List (SFNSL). Patients reported improvements in both pain and autonomic symptoms.
- Corneal Nerve Fiber Regeneration: In clinical studies, ARA-290 treatment was associated with an increase in corneal nerve fiber density (CNFD) in patients with reduced CNFD at baseline, suggesting a potential for nerve regeneration.
- Metabolic Control in Type 2 Diabetes: A phase 2 study in patients with type 2 diabetes showed that ARA-290 improved hemoglobin A1c levels and lipid profiles, indicating a potential benefit for metabolic control.
- Anti-inflammatory Effects: Research has shown that ARA-290 can reduce the activation of microglia and astrocytes in the spinal cord following nerve injury, suggesting an anti-inflammatory mechanism of action.
Future Research Directions
- Long-term Safety and Efficacy: Extended studies are needed to evaluate the long-term effects and safety profile of ARA-290 in various patient populations.
- Mechanism of Action: Further research is required to fully elucidate the molecular mechanisms by which ARA-290 exerts its diverse effects, particularly in different tissue types.
- Combination Therapies: Exploring the potential synergistic effects of ARA-290 with other therapeutic agents could open new avenues for treatment in various medical fields.
- Expanded Clinical Applications: Investigation into the potential benefits of ARA-290 in other conditions characterized by tissue damage or chronic inflammation is warranted.
- Optimal Dosing Regimens: Research into optimal delivery methods and dosing regimens for different conditions could enhance the therapeutic potential of ARA-290.
References
-
Brines, M., et al. (2014). ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Molecular Medicine, 20(1), 658-666. [Online].
Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4365069/ -
Heij, L., et al. (2012). Safety and Efficacy of ARA 290 in Sarcoidosis Patients with Symptoms of Small Fiber Neuropathy: A Randomized, Double-Blind Pilot Study. Molecular Medicine, 18, 1430–1436. [Online].
Available https://molmed.biomedcentral.com/articles/10.2119/molmed.2012.00332 -
Dahan, A., et al. (2016). Targeting the innate repair receptor to treat neuropathy. Pain Reports, 1(1), e566. [Online].
Available: https://journals.lww.com/painrpts/fulltext/2016/08000/targeting_the_innate_repair_receptor_to_treat.2.aspx -
Lois, N., et al. (2020). A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema. Journal of Clinical Medicine, 9(7), 2225. [Online].
Available: https://www.mdpi.com/2077-0383/9/7/2225 -
Brines, M., et al. (2014). ARA 290, a Nonerythropoietic Peptide Engineered from Erythropoietin, Improves Metabolic Control and Neuropathic Symptoms in Patients with Type 2 Diabetes. Molecular Medicine, 20(1), 658-666. [Online]. .
Available: https://molmed.biomedcentral.com/articles/10.2119/molmed.2014.00215 -
Dahan, A., et al. (2013). ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Molecular Medicine, 19(1), 334-345. [Online].
Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3883966/


