Product Usage: This product is designated as a "Novelty Peptide" and is intended solely for specialized applications in controlled settings. It is not intended for any specific biological, therapeutic, or diagnostic use. All information provided on this website is for informational and educational purposes only and should not be construed as guidance for any particular application or usage.
What Is Tesamorelin 50MG?
Tesamorelin is a synthetic analog of growth hormone-releasing hormone (GHRH) that stimulates the production and release of growth hormone from the pituitary gland. It is FDA-approved for the treatment of HIV-associated lipodystrophy, specifically to reduce excess abdominal fat in HIV-infected patients. Tesamorelin has shown promise in addressing metabolic issues, improving body composition, and potentially offering benefits for conditions like non-alcoholic fatty liver disease (NAFLD) in HIV patients.
Structure
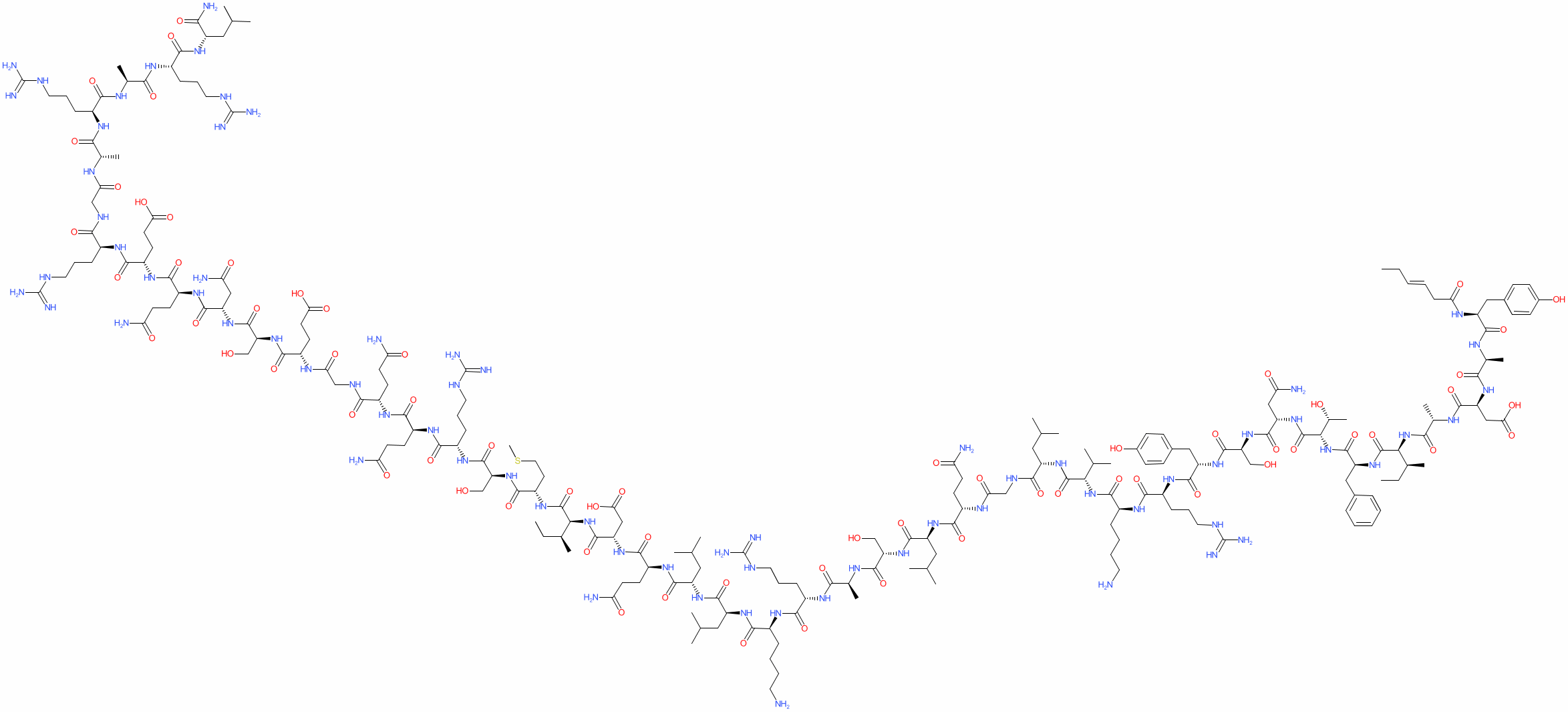
Sequence: Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu-NH2
Molecular Formula: C221H366N72O67S
Molecular Weight: 5135.9 g/mol
CAS Number: 901758-09-6
Research Findings
- Visceral Fat Reduction: Clinical trials have demonstrated that tesamorelin significantly reduces visceral adipose tissue (VAT) in HIV-infected patients with lipodystrophy. A pooled analysis of two phase-3 studies showed a 15.4% treatment effect in VAT reduction after 26 weeks of treatment.
- Liver Fat Reduction: Tesamorelin has shown efficacy in reducing liver fat in HIV patients with non-alcoholic fatty liver disease (NAFLD). A study reported a significant decrease in hepatic fat fraction in the tesamorelin group compared to placebo.
- Metabolic Improvements: Treatment with tesamorelin has been associated with improvements in lipid profiles, including reductions in triglycerides and total cholesterol to HDL ratio.
- Fibrosis Prevention: In HIV patients with NAFLD, tesamorelin demonstrated the ability to prevent progression of liver fibrosis, with strong relationships observed between reductions in liver fat and fibrosis.
- Body Image: Tesamorelin treatment has been reported to improve body image and reduce distress related to abdominal appearance in HIV patients with lipodystrophy.
Future Research Directions
- Long-term Safety and Efficacy: Extended studies are needed to evaluate the long-term effects and safety profile of tesamorelin, particularly in diverse patient populations and for extended use beyond current approval periods.
- Expanded Applications: Further research is warranted to explore tesamorelin's potential in treating NAFLD/NASH in non-HIV populations, as well as its effects on other metabolic disorders.
- Cognitive Function: Preliminary studies have suggested potential cognitive benefits of tesamorelin in healthy older adults and those with mild cognitive impairment. Further investigation in this area is needed.
- Combination Therapies: Exploring potential synergistic effects of tesamorelin with other therapeutic agents could open new avenues for treatment in various medical fields.
- Mechanism of Action: Additional studies to fully elucidate the molecular mechanisms by which tesamorelin exerts its diverse effects across different tissue types are needed.
References
-
BMI Doctors. (2024). Tesamorelin Unveiled: Its Impact in Medical Science. [Online].
Available: https://bmidoctors.com/tesamorelin-unveiled-its-impact-in-medical-science/ -
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. (2018). Tesamorelin. [Online].
Available: https://www.ncbi.nlm.nih.gov/books/NBK548730/ -
Stanley, T.L., et al. (2014). Effect of Tesamorelin on Visceral Fat and Liver Fat in HIV-Infected Patients With Abdominal Fat Accumulation: A Randomized Clinical Trial. JAMA, 312(4), 380-389. [Online].
Available: https://jamanetwork.com/journals/jama/fullarticle/1889139 -
MedlinePlus. (2016). Tesamorelin Injection. [Online].
Available: https://medlineplus.gov/druginfo/meds/a611035.html -
Falutz, J., et al. (2007). Metabolic Effects of a Growth Hormone–Releasing Factor in Patients with HIV. New England Journal of Medicine, 357(23), 2359-2370. [Online].
Available: https://www.nejm.org/doi/full/10.1056/NEJMoa072375 -
LIVV Natural. (2024). Tesamorelin and Gender in Hormone Therapies. [Online].
Available: https://livvnatural.com/tesamorelin-and-the-gender-debate-should-hormone-therapies-be-gender-specific/ -
Falutz, J., et al. (2010). Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. Journal of Clinical Endocrinology & Metabolism, 95(9), 4291-4304. [Online].
Available: https://pubmed.ncbi.nlm.nih.gov/20554713/ -
Stanley, T.L., et al. (2019). Effects of Tesamorelin on Non-alcoholic Fatty Liver Disease in HIV: A Randomised, Double-Blind, Multicentre Trial. The Lancet HIV. 6(12), e821-e830. [Online]. .
Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6981288/


